"Diamondoid bacteria" nanobots: deadly threat or dead-end? A nanotech investigation
Confidence level: I’m a computational physicist working on nanoscale simulations, so I have some understanding of most of the things discussed here, but I am not specifically an expert on the topics covered, so I can’t promise perfect accuracy.
I want to give a huge thanks to Professor Phillip Moriarty of the university of Nottingham for answering my questions about the experimental side of mechanosynthesis research.
Introduction:
A lot of people are highly concerned that a malevolent AI or insane human will, in the near future, set out to destroy humanity. If such an entity wanted to be absolutely sure they would succeed, what method would they use? Nuclear war? Pandemics?
According to some in the x-risk community, the answer is this: The AI will invent molecular nanotechnology, and then kill us all with diamondoid bacteria nanobots.
This is the “lower bound” scenario posited by Yudkowsky in his post AGI ruin:
The nanomachinery builds diamondoid bacteria, that replicate with solar power and atmospheric CHON, maybe aggregate into some miniature rockets or jets so they can ride the jetstream to spread across the Earth's atmosphere, get into human bloodstreams and hide, strike on a timer.
The phrase “diamondoid bacteria” really struck out at me, and I’m not the only one. In this post by Carlsmith (which I found very interesting), Carlsmith refers to diamondoid bacteria as an example of future tech that feels unreal, but may still happen:
Whirling knives? Diamondoid bacteria? Relentless references to paper-clips, or “tiny molecular squiggles”? I’ve written, elsewhere, about the “unreality” of futurism. AI risk had a lot of that for me.
Meanwhile, the controversial anti-EA crusader Emille Torres cites the term “diamondoid bacteria” as a reason to dismiss AI risk, calling it “patently ridiculous”.
I was interested to know more. What is diamondoid bacteria? How far along is molecular nanotech research? What are the challenges that we (or an AI) will need to overcome to create this technology?
If you want, you can stop here and try and guess the answers to these questions.
It is my hope that by trying to answer these questions, I can give you a taste of what nanoscale research actually looks like. It ended up being the tale of a group of scientists who had a dream of revolutionary nanotechnology, and tried to answer the difficult question: How do I actually build that?
What is “diamondoid bacteria”?
The literal phrase “diamondoid bacteria” appears to have been invented by Eliezer Yudkowsky about two years ago. If you search the exact phrase in google scholar there are no matches:
If you search the phrase in regular google, you will get a very small number of matches, all of which are from Yudkowsky or directly/indirectly quoting Yudkowsky. The very first use of the phrase on the internet appears to be this twitter post from September 15 2021. (I suppose there’s a chance someone else used the phrase in person).
I speculate here that Eliezer invented the term as a poetic licence way of making nanobots seem more viscerally real. It does not seem likely that the hypothetical nanobots would fit the scientific definition of bacteria, unless you really stretched the definition of terms like “single-celled” and “binary fission”. Although bacteria are very impressive micro-machines, so I wouldn’t be surprised if future nanotech bore at least some resemblance.
Frankly, I think inventing new terms is an extremely unwise move (I think that Eliezer has stopped using the term since I started writing this, but others still are). “diamondoid bacteria” sounds science-ey enough that a lot of people would assume it was already a scientific term invented by an actual nanotech expert (even in a speculative sense). If they then google it and find nothing, they are going to assume that you’re just making shit up.
But diamondoid nanomachinery has been a subject of inquiry, by actual scientific experts, in a research topic called “diamondoid mechanosynthesis”.
What is “diamondoid mechanosynthesis”
Molecular nanotech (MNT) is an idea first championed by Eric Drexler, that the same principles of mass manufacturing that are used in todays factories could one day be miniaturized to the nanoscale, assembling complex materials molecule by molecule from the ground up, with nanoscale belts, gears, and manipulators. You can read the thesis here, It’s an impressive first theoretical pass at the nanotech problem, considering the limited computational tools available in 1991, and helped inspire many in the current field of nanotechnology (which mostly does not focus on molecular assembly).
However, Drexlers actual designs of how a molecular assembler would be built have been looked on with extreme skepticism by the wider scientific community. And while some of the criticisms have been unfair (such as accusations of pseudoscience), there are undeniably extreme engineering challenges. The laws of physics are felt very differently at different scales, presenting obstacles that have never been encountered before in the history of manufacturing, and indeed may turn out to be entirely insurmountable in practice. How would you actually make such a device?
Well, a few teams were brave enough to try and tackle the problem head on. The nanofactory collaboration, with a website here, was an attempt to directly build a molecular assembler. It was started in the early 2000’s, with the chief players beings Freitas and Merkle, two theoretical/computational physicists following on from the work of Drexler. The method they were researching to make this a reality was diamondoid mechanosynthesis(DMS).
So, what is DMS? Lets start with Mechanosynthesis. Right now, if you want to produce molecules from constituent molecules or elements, you would place reactive elements in a liquid or gas and jumble them around so they bump into each other randomly. If the reaction is thermodynamically favorable under the conditions you’ve put together (temperature, pressure, etc.), then mass quantities of the desired products are created.
This is all a little chaotic. What if we wanted to do something more controlled? The goal of mechanosynthesis is to precisely control the reactive elements we wish to put together by using mechanical force to precisely position them together. In this way, the hope is that extremely complex structures could be assembled atom by atom or molecule by molecule.
The dream, as expressed in the molecular assembler project, was that mechanosynthesis can be mastered to such a degree that “nano-factories” could be built, capable of building many different things from the ground up, including another nanofactory. If this could be achieved, then as soon as one nanofactory is built, a vast army of them would immediately follow through the power of exponential growth. These could then build nanomachines that move around, manipulate objects, and build pretty much anything from the ground up, like a real life version of the Star Trek matter replicator.
If you want to convert a dream into a reality, you have to start thinking of engineering, If you could make such a nano-factory, what would it be made out of? There are a truly gargantuan number of materials out there we could try out, but almost all of them are not strong enough to support the kind of mechanical structures envisaged by the nanofactory researchers. The most promising candidate was “diamondoid”.
Now, what is “diamondoid”? You’d expect this to be an easy question to answer, but it’s actually a little thorny. The more common definition, the one used on wikipedia and most journal papers, is that diamondoid refers to a specific family of hydrocarbons like the ones shown below, with the simplest one being “adamantane”, with it’s strong, cage-like structure, and the other ones being formed by joining together multiple cages.

These cages are incredibly strong and stable, which makes them a promising candidate material for building up large structures, and keeping them stable for assembly purposes.
The other definition, which seems to be mainly used by the small community of molecular nanotech(MNT) proponents, is that “diamondoid” just means “any sufficiently strong and stiff nanoscale material”. See this passage from the “molecular assembler” website:
Diamondoid materials also may include any stiff covalent solid that is similar to diamond in strength, chemical inertness, or other important material properties, and possesses a dense three-dimensional network of bonds. Examples of such materials are carbon nanotubes (illustrated at right) or fullerenes, several strong covalent ceramics such as silicon carbide, silicon nitride, and boron nitride, and a few very stiff ionic ceramics such as sapphire (monocrystalline aluminum oxide) that can be covalently bonded to pure covalent structures such as diamond.
This passage is very out of line with mainstream definitions. I couldn’t find a mention of “diamondoid” in any top carbon nanotube article. I’ve done a little research on aluminium oxide, and I have never in my life heard it called “diamondoid”, considering it neither contains the same elements as diamond, nor does it take the same structure as diamond or diamondoid hydrocarbons. This kind of feels like the “radical sandwich anarchy” section of this chart.
I really don’t want to get sidetracked into semantic debates here. But just know that the MNT definition is non-standard, might annoy material scientists, and could easily be used against you by someone with a dictionary.
In any case, it’s not a huge deal, because the molecular assembler team was focused on carbon-based diamond and diamondoid structures anyway.
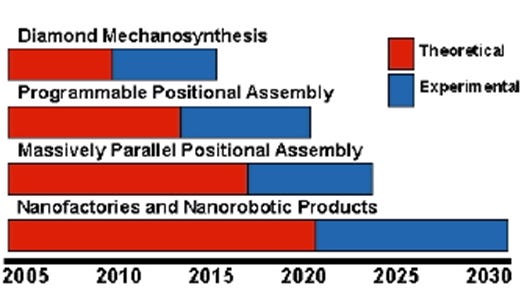
The plan was to engage in both theoretical and experimental research to develop nanotech in several stages. Step 1 was to achieve working prototypes of diamond mechanosynthesis. Step 2 was to build on this to actually assemble complex molecular structures in a programmable mechanical manner. Step 3 was to find a way to parallelize the process, so that huge amounts of assembly could be done at once. Step 4 was to use that assembly to build a nanofactory, capable of building a huge number of things, including a copy of itself. The proposed timeline for this project is shown below:
They thought they would have the first three steps finished by 2023, and have working commercialized nanofactories by 2030. Obviously, this is not on track. I’m not holding this against them, as extremely ambitious projects rarely finish on schedule. They were also underfunded compared to what they wanted, furthering hampering progress.
How far did the project go, in the end?
DMS research: The theoretical side
The nanofactory collaboration put forward a list of publications, and as far as I can tell, every single one is theoretical or computational in nature. There are a few book chapters and patent applications, as well as about a dozen peer-reviewed scientific articles, mostly in non-prestigious journals1.
Skimming through the papers, they seem fine. A lot of time and effort has gone into them, I don’t see any obvious problems with their methodology, and the reasoning and conclusions seem to be a reasonable. Going over all of them would take way too long, but I’ll just pick one that is representative and relatively easy to explain: “Theoretical Analysis of Diamond Mechanosynthesis. Part II. C2 Mediated Growth of Diamond C(110) Surface via Si/Ge-Triadamantane Dimer Placement Tools”.
Please don’t leave, I promise you this is interesting!
The goal of this paper is simple: we want to use a tooltip to pick up a pair of carbon atoms (referred to as a “dimer”), place the dimer on a carbon surface (diamond), and remove the tooltip, leaving the dimer on the surface.
In our large world, this type of task is pretty easy: you pick up a brick, you place it where you want, and then you let it go. But all the forces present at our scale are radically different at the nanoscale. For example, we used friction to pick the brick up, but “friction” does not really exist at the single atom scale. Instead, we have to bond the cargo element to our tool, and then break that bond at the right moment. It’s like if the only way to lay bricks was to glue your hand to a brick, glue the brick to the foundation, and then rip your hand away.
Below we have the design for their tooltip that they were investigating here. We have our diamondoid cages from earlier, but we replace a pair of corner atoms with Germanium (or Si) atoms, and bond the cargo dimer to these corners, in the hopes it will make them easier to detach:
The first computational result is a checking of this structure using DFT simulations. I have described DFT and it’s strengths and shortcomings in this previous post. They find that the structure is stable in isolation.
Okay great, it’s stable on it’s own, but the eventual plan is to have a whole ton of these around working in parallel. So the next question they ask is this: if I have a whole bunch of these together, are they going to react with each other and ruin the tooltip? The answer, they find, is yes, in two different ways. Firstly, if two of these meet dimer-to-dimer, it’s thermodynamically favorable for them to fuse together into one big, useless tooltip. Secondly, if one encounters the hydrogen atoms on the surface of the other, it would tear them out to sit on the end of the cargo dimer, rendering it again useless. They don’t mention it explicitly, but I assume the same thing would happen if it encountered stray hydrogen in the air.
This is a blow to the design, and would mean great difficulty in actually using the thing large scale. In theory you could still pull it off by keeping the tools isolated from each other.
They check the stability of the tooltip location itself using molecular dynamics calculation, and find that it’s stable enough for purpose, with a stray that is smaller than the chemical bond distances involved.
And now for the big question: can it actually deposit the dimer on the surface? The following graph summarizes the DFT results:
On the left side, we have the initial state. The tooltip is carrying the cargo dimer. At this step, and at every other, a DFT calculation is taken out to calculate the entire energy of the simulation.
In the middle, we have the middle state. The tooltip has been lowered, carrying the tooltip to the surface, where the carbon dimer is now bonded both to the tooltip and to the diamond surface.
On the right, we have the desired final state. The tooltip has been retracted and raised, but the carbon is left behind on the surface.
All three states have been simulated using DFT to predict their energy, and so have a number of intermediate steps in between. From this, we can see that the middle step is predicted to be 3 eV more energetically favorable than the left state, meaning that there will be no problem progressing from left to middle.
The real problem they find is in going from the middle state to the right state. There is about a 5 eV energy barrier to climb to remove the tooltip. This is not a game ender, as we can apply such energy mechanically by pulling on the tooltip (I did a back of the envelope calculation and the energy cost didn’t seem prohibitive2).
No, the real problem is that when you pull on the tooltip, there no way to tell it to leave the dimer behind on the surface. In fact, it’s lower energy to rip up the carbon dimer as well, going right back to the left state, where you started.
They attempt a molecular dynamics simulation, and found that with the Germanium tip, deposition failed 4 out of 5 times (for silicon, it failed every time). They state this makes sense because the extra 1 eV barrier is small enough to be overcome, at least some of the time, by 17eV of internal (potential+kinetic) energy. If I were reviewing this paper I would definitely ask for more elaboration on these simulations, and where exactly the 17 eV figure comes from. They conclude that while this would not be good enough for actual manufacturing, it’s good enough for a proof of concept.
In a later paper, it is claimed that the analysis above was too simplistic, and that a more advanced molecular dynamics simulation shows the Ge tool reliably deposits the dimer on the surface every time. It seems very weird and unlikely to me that the system would go to the higher energy state 100% of the time, but I don’t know enough about how mechanical force is treated in molecular dynamics to properly assess the claim.
I hope that this analysis has given you a taste of the type of problem that is tackled in computational physics, and how it is tackled. From here, they looked at a few other challenges, such as investigating more tip designs, looking at the stability of large diamondoid structures, and a proposed tool to remove hydrogen from a surface in order to make it reactive, a necessary step in the process.
Experimental diamondoid research
Recall that the goal of this theoretical research was to set the stage for experimental results, with the eventual goal of actually building diamondoid. But if you look at the collaborators of the project, almost everyone was working on theory. Exactly one experimentalist team worked on the project.
The experimentalist in question was university of Nottingham professor Phillip Moriarty, of sixty symbols fame (he has a blog too). Interestingly enough, the collaboration was prompted by a debate with an MNT proponent in 2004, with Moriarty presenting a detailed skeptical critique of DMS proposals and Drexler-style nanotech in general. A sample of his concerns:
While I am open to the idea of attempting to consider routes towards the development of an implementation pathway for Mann et al.’s Si/Ge-triadamantane dimer placement reaction, even this most basic reaction in mechanochemistry is practically near-impossible. For example, how does one locate one tool with the other to carry out the dehydrogenation step which is so fundamental to Mann et al.’s reaction sequence?
….
Achieving a tip that is capable of both good atomic resolution and reliable single molecule positioning (note that the Nottingham group works with buckyballs on surfaces of covalently bound materials (Si(111) and Si(100)) at room temperature) requires a lot of time and patience. Even when a good tip is achieved, I’ve lost count of the number of experiments which went ‘down the pan’ because instead of a molecule being pushed/pulled across a surface it “decided” to irreversibly stick to the tip.
Despite the overall skepticism, he approved of the research efforts by Freitas et al, and the correspondence between them led to Moriarty signing on to the nanofactory project. Details on what happened next are scarce on the website.
Rather than try and guess what happened, I emailed Moriarty directly. The full transcripts are shown here.
Describing what happened, Moriarty explained that the work on diamond mechanosynthesis was abandoned after ten months:
Diamond is a very, very difficult surface to work with. We spent ten months and got no more than a few, poorly resolved atomic force microscopy (AFM) images. We’re not alone. This paper -- https://journals.aps.org/prb/cited-by/10.1103/PhysRevB.81.201403 (also attached)-- was the first to show atomic resolution AFM of the diamond surface. (There’d previously been scanning tunnelling microscopy (STM) images and spectroscopy of the diamond (100) surface but given that the focus was on mechanical force-driven chemistry (mechanosynthesis), AFM is a prerequisite.) So we switched after about a year of that project (which started in 2008) to mechanochemistry on silicon surfaces – this was much more successful, as described in the attached review chapter.
Inquiring as to why diamond was so hard to work with, he replied:
A key issue with diamond is that tip preparation is tricky. On silicon, it’s possible to recover atomic resolution relatively straight-forwardly via the application of voltage pulses or by pushing the tip gently (or not so gently!) into the surface – the tip becomes silicon terminated. Diamond is rather harder than silicon and so once the atomistic structure at the end is lost, it needs to be moved to a metal sample, recovered, and then moved back to the diamond sample. This can be a frustratingly slow process.
Moreover, it takes quite a bit of work to prepare high quality diamond surfaces. With silicon, it’s much easier: pass a DC current through the sample, heat it up to ~ 1200 C, and cool it down to room temperature again. This process routinely produces large atomically flat terraces.
So it turns out that mechanosynthesis experiments on diamond are hard. Like ridiculously hard. Apparently only one group ever has managed to successfully image the atomic surface in question. This renders attempts to do mechanosynthesis on diamond impractical, as you can’t tell whether or not you’ve pulled it off or not.
This is a great example of the type of low-level practical problem that is easy to miss if you are a theoretician (and pretty much impossible to predict if you aren’t a domain expert).
So all of those calculations about the best tooltip design for depositing carbon on diamond ended up being completely useless for the problem of actually building a nanofactory, at least until imaging technology or techniques improve.
But there wasn’t zero output. The experimental team switched materials, and was able to achieve some form of mechanosynthesis. It wasn’t on diamond, but Silicon, which is much easier to work with. And it wasn’t deposition of atoms, it was a mechanical switch operated with a tooltip, summarized in this youtube video. Not a direct step toward molecular assembly, but still pretty cool.
As far as I can tell, that’s the end of the story, when it comes to DMS. The collaboration appears to have ended in the early 2010’s, and I can barely find any mention of the topic in the literature past 2013. They didn’t reach the dream of a personal nanofactory: they didn’t even reach the dream of depositing a few carbon atoms on a diamond surface.
A brief defense of dead research directions
I would say that DMS research is fairly dead at the moment. But I really want to stress that that doesn’t mean it was bad research, or pseudoscience, or a waste of money.
They had a research plan, some theoretical underpinnings, and explored a possible path to converting theory into experimental results. I can quibble with their definitions, and some of their conclusions seem overly optimistic, but overall they appear to be good faith researchers making a genuine attempt to expand knowledge and tackle a devilishly difficult problem with the aim of making the world a better place. That they apparently failed to do so is not an indictment, it’s just a fact of science, that even great ideas mostly don’t pan out into practical applications.
Most research topics that sound good in theory don’t work in practice, when tested and confronted with real world conditions. This is completely fine, as the rare times when something works, a real advancement is made that improves the lives of everyone. The plan for diamondoid nanofactories realistically had a fairly small chance of working out, but if it had, the potential societal benefits could have been extraordinary. And the research, expertise, and knowledge that comes out of failed attempts are not necessarily wasted, as they provide lessons and techniques that help with the next attempt.
And while DMS research is somewhat dead now, that doesn’t mean it won’t get revived. Perhaps a new technique will be invented that allows for reliable imaging of diamondoid, and DMS ends up being successful eventually. Or perhaps after a new burst of research, it will prove impractical again, and the research will go to sleep again. Such is life, in the uncertain realms of advanced science.
Don’t worry, nanotech is still cool as hell
At this point in my research, I was doubting whether even basic nanomachines or rudimentary mechanosynthesis was even possible. But this was an overcorrection. Nanoscience is still chuggin along fine. Here, I’m just going to list a non-exhaustive list of some cool shit we have been able to do experimentally. (most of these examples were taken from “nanotechnology: a very short introduction”, written by Phillip Moriarty (the same one as before).
First, I’ll note that traditional chemistry can achieve some incredible feats of engineering, without the need for mechanochemistry at all. For example, in 2003 the Nanoputian project successfully built a nanoscale model of a person out of organic molecules. They used cleverly chosen reaction pathways to produce the upper body, and cleverly chosen reaction pathways to produce the lower body, and then managed to pick the exact right conditions to mix them together in that would bond the two parts together.
Similarly, traditional chemistry has been used to build “nanocars” , nanoscale structures that contain four buckyball wheels connected to a molecular “axle”, allowing it to roll across a surface. Initially, these had to be pushed directly by a tooltip. In later versions, such as the nanocar race, the cars are driven by electron injection or electric fields from the tooltip, reaching top speeds of 300 nm per hour. Of course, at this speed the nanocar would take about 8 years to cross the width of a human finger, but it’s the principle that counts.
The Nobel prize in 2016 was awarded to molecular machines, for developing molecular lifts, muscles, and axles.
I’ll note that using a tooltip to slide atoms around has been a thing since 1990, when IBM wrote their initials using xenon atoms. A team achieved a similar feat for selected silicon atoms on silicon surfaces in 2003, using purely mechanical force.
As for the dream of molecular assembly, the goal of picking atoms up and placing them down has been achieved by a UK team, which were able to use a chemical arm to pick up a cargo molecule bonded on one side, transfer it to another side, and drop it and leave it in place:
This is not mechanosynthesis as it is not powered by direct mechanical force, but from chemical inputs, such as varying the acidity of the solution. It is also based on more complex organic molecules, rather than diamondoid structures.
This brings us to what seems the most interesting and promising area : DNA based nanotech. This makes sense: over billions of years evolution already figured out a way to build extremely complex self-replicating machines, which can also build little bots as small as 20nm across. Actual bacteria are larger scale and more fragile than hypothetical nanofactories, but have the distinct advantage of actually existing. Why reinvent the wheel?
I have very little background in biology, so I won’t venture too deeply into the topic (which deserves a whole post of it’s own), but there have been a number of highly impressive achievements in DNA based nanotech. The techniques of DNA origami allow for DNA structures to fold up among themselves to form a variety of structures, such as spheres, cubes, and nanoflasks. One team used one such DNA nanorobot to target tumour growth in mice. The research is still some ways from practical human applications (and many such promising medical technologies end up being impractical anyway). Nonetheless, I’m impressed, and will be watching this space closely.
So are diamondoid bots a threat?
It’s very hard to prove that a technology won’t pan out, if it doesn’t inherently break the laws of physics. But a tech being “not proven to be impossible” does not mean the tech is “inevitable”.
With regards to diamondoid specifically, the number of materials that are not diamondoid outnumbers the number of materials that are by a truly ridiculously large margin. And although diamondoid has a lot going for it in terms of stiffness and strength, we saw that it also has shortcomings that make it difficult to work with, and potential minefields like the tooltips theoretically sticking to each other. So my guess is that if Drexler-style nanofactories are possible, they will not be built up of diamondoid.
How about nanofactories made of other materials? Well, again, there are a truly gargantuan number of materials available, which does give some hope. But then, this is also a ridiculously hard problem. We haven’t even scratched the surface of the difficulties awaiting such a project. Depositing one measly dimer on a surface turned out to be too hard, but once we achieved that, you have to figure out how to place the next one, and the next one, and build a proper complex structure without getting your tooltip stuck. You need a way to harvest your sources of carbon to build things up with. If you want to be truly self-sufficient and self-replicating, you need a source of energy for the mechanical force needed to rip atoms away, and a means of propulsion to move your entire nanofactory around.
Designs have been proposed for a lot of these problems (like in Drexlers thesis), but each step is going to be beset with endless issues and engineering challenges that would have to be trudged through, one step at a time. We’ve barely gotten to step 1.
Fusion power is often accurately mocked for having been “20 years away” for over three decades. It had proofs of concept and was understood, it seemed that all was left was the engineering, which ended up being ridiculously hard. To me, molecular nanotech looks about 20 years away from being “20 year away”. At the current rate of research, I would guess it won’t happen for at least 60 years, if it happens at all. I would be happy to be proven wrong.
I consulted professor Moriarty whether he thought the scenario proposed by Yudkowsky was plausible:
We are a long, long, loooong way from the scenario Yudkowsky describes. For example, despite it being 33 years since the first example of single atom manipulation with the STM (the classic Eigler and Schweizer Nature paper where they wrote the IBM logo in xenon atoms), there’s yet to be a demonstration of the assembly of even a rudimentary 3D structure with scanning probes: the focus is on the assembly of structures by pushing, pulling, and/or sliding atoms/molecules across a surface. Being able to routinely pick up, and then drop, an atom from a tip is a much more complicated problem.
Marauding swarms of nanobots won’t be with us anytime soon.
This seems like a good place to note that MNT proponents have a record of extremely over-optimistic predictions. See this estimation of MNT arrival from Yudkowsky in 1999:
As of '95, Drexler was giving the ballpark figure of 2015 (11). I suspect the timetable has been accelerated a bit since then. My own guess would be no later than 2010.
Could the rate of research accelerate?
Now, I can’t leave without addressing the most likely objection. I said greater than 60 years at the current rate of research. But what if the rate of research speeds up?
One idea is that the DNA or bio-based robots will be used to build a drexler-style nanofactory. This is the “first stage nanofactory” that yud mentions in list of lethalities, and it was the first step proposed by Drexler as well. I see how this could enable better tools and more progress, but I’m not sure how this would affect the fundamental chemistry issues that need to be overcome to build a non-protein based machine. How will the biobot stop two tooltips from sticking together?. If you want to molecularly assemble something, would in really be better for a tooltip to be held by a wiggly biologically based bot, instead of a precisely computerized control tooltip?
The more common objection is that artifical intelligence will speed this research up. Well, now we’re working with two high uncertainty, speculative technologies. To keep this simple I’ll restrict this analysis to the short term (the next decade or so), and assume no intelligence explosion occurs. I might revisit the subject in more depth later on.
First, forget the dream of advances in theory rendering experiment unnecessary. As I explained in a previous post, the quantum equations are just way too hard to solve with 100% accuracy, so approximations are necessary, which themselves do not scale particularly well.
Machine learning in quantum chemistry has been investigated for some time now, and there are promising techniques that could somewhat speed up a subset of calculations, and make some larger-scale calculations feasible that were not before. For my research, the primary speedups from AI come from using chatGPT to speed up coding a bit and helping to write bureaucratic applications.
I think if the DMS project were ran today, the faster codes would allow for slightly more accurate results, more calculations per paper allowing for more materials to be investigated, and potentially the saved time from writing and coding could allow for another few papers to be squeezed out. For example, if they used the extra time to look at silicon DMS as well as carbon DMS, they might have gotten something that could actually be experimentally useful.
I’m not super familiar with the experimental side of things. In his book, Moriarty suggests that machine learning could be applied to:
image and spectral classification in various forms of microscopy, automation of time-consuming tasks such as optimization of the tip of an STM or AFM, and the positioning of individual atoms and molecules.
So I think this could definitely speed up parts of the experimental process. However, there are still going to be a lot of human-scale bottlenecks to keep a damper on things, such as sample preparation. And as always with practical engineering, a large part of the process will be figuring out what the hell went wrong with your last experiment. There still is no AI capable of figuring out that your idiot labmate Bob has been contaminating your samples by accident.
What about super-advanced AGI? Well, now we’re guessing about two different speculative technologies at once, so take my words (and everyone else’s) with a double grain of salt. Obviously, an AGI would speed up research, but I’m not sure the results would be as spectacular as singularity theorists expect.
An AI learning, say, Go, can play a hundred thousand games a minute with little difficulty. In science, there are likely to be human-scale bottlenecks that render experimentation glacial in comparison. High quality quantum chemistry simulations can take days or weeks to run, even on supercomputing clusters. On the experimental side humans have to order parts, deliver them, prepare the samples, maintain the physical equipment, etc. It’s possible that this can be overcome with some sort of massively automated robotic experimentation system… but then you have to build that system, which is a massive undertaking in itself. Remember, the AI would not be able to use MNT to build any this. And of course, this is all assuming the AI is actually competent, and that MNT is even possible in practicality.
Overall, I do not think trying to build drexler-style nanomachinery would be an effective plan for the adversaries of humanity, at least as things currently stand. If they try, I think we stand a very good chance of detecting and stopping them, if we bother to look instead of admitting premature defeat.
Summary
“Diamondoid bacteria” is a phrase that was invented 2 years ago, referring obliquely to diamondoid mechanosynthesis (DMS)
DMS refers to a proposed technology where small cage-like structures are used to position reactive molecules together to assemble complex structures.
DMS research was pursued by a nanofactory collaboration of scientists starting in the early 2000’s.
The theoretical side of the research found some potentially promising designs for the very initial stages of carbon deposition but also identified some major challenges and shortcomings.
The experimental side was unsuccessful due to the inability to reliably image the diamond surface.
Dead/stalled projects are not particularly unusual and should not reflect badly on the researchers.
There are still many interesting advances and technologies in the field of nanoscience, including promising advances in DNA based robotics.
At the current rate of research, DMS style nanofactories still seem many, many decades away at the minimum.
This research could be sped up somewhat by emerging AI tech, but it is unclear to what extent human-scale bottlenecks can be overcome in the near future.
Scientific journals are generally judged on their average number of citations per paper (impact factor). Most of the papers were published in a journal with the fairly low IF of 0.4. Low IF is correlated with less scrutiny and rigor, but I wouldn’t put too much weight on it. Plus, the whole for-profit journal system is dogshit.
If it took 5 eV to place a carbon dimer, and this was the only limitation, then assembling a cubic centimeter of diamond would use about 70 kJ of energy, roughly equivalent to four 9 volt batteries. Lab grown diamonds use about 25 kwH per carat which would be about a 1.5 million kJ of energy per cubic centimeter.





>For example, in 2003 the Nanoputian project successfully built a nanoscale model of a person out of organic molecules. They used cleverly chosen reaction pathways to produce the upper body, and cleverly chosen reaction pathways to produce the lower body, and then managed to pick the exact right conditions to mix them together in that would bond the two parts together
As a chemist by training, I don't think this is actually that impressive. They basically did a few Sonogashira couplings, which are rather easy reactions (I did them regularly as an undergrad).
If you want something impressive, look at the synthesis of vitamin B12: https://en.wikipedia.org/wiki/Vitamin_B12_total_synthesis
Also . . . you don't need "diamondoid" technology to make nano-replicators that kill everything. Highly engineered bacteria could do the trick.
The Nanofactory collaboration didn't die out in 2017, they just went underground. They got acquired by the Canadian Bank Note corporation, which provided some internal funding alongside a $40M CAD grant from the Canadian government (SIF loan 813022). Their patent applications make for interesting reading: https://www.onscope.com/ipowner/en/owner/profile/1827206-cbn-nano-technologies-inc.html
There are other people working on direct-to-diamondoid mechanosynthesis as well, so the field is hardly dead.